Background
The NCRI FLAIR trial confirmed ibrutinib + rituximab (IR) improved progression free survival (PFS) over FCR in untreated CLL. Durable responses are achieved on continuous Bruton's tyrosine kinase inhibitor (BTKi) therapy, but acquired resistance mutations in BTK, and PLCG2 are reported in up to 80% of relapsed/refractory patients (pts) failing BTKi often detected at low variant allele frequencies (VAF) several months before relapse. Their frequency, evolutionary dynamics, and relevance to progression in the upfront-treatment setting are poorly characterised. We present the observed clonal evolution in standard risk pts with disease progression (DP) on IR, integrating next generation sequencing (NGS) with serial measurable disease monitoring by flow cytometry, and tracking individual BTK mutations ( BTKmt) by digital droplet PCR (ddPCR) from initial detection to DP.
Methods
771 randomised pts to FCR (n=385) or IR (n=386). Pts treated with IR received rituximab at 375 mg/m 2 on D1/C1 and 500 mg/m 2 on D1/C 2-6 of every 28-days. I was given orally at 420 mg/day for up to 72 mo. All 61 DP pts on IR were included in the analysis. DNA from baseline and progression peripheral blood (PB) samples were processed for sequencing of 33 genes recurrently mutated in lymphoid malignancies including BTK (exon 15) and PLCG2 (exons 19-20) with an Illumina MiSeq. Variants were reported down to minimum VAF of 3-5% and coverage of 100X. Low VAF (3-6%) BTKmt were validated by ddPCR using the QX200 ddPCR protocol (sensitivity 1-0.1%). ddPCR was used for the individual serial analysis at baseline and 6 monthly in pts with confirmed BTKhot spot mutations (p.C481S (c.1441T>A, c.1442G>C)), p.C481R (c.1441T>C), p.T474I (c.1421C>T) and presented with concurrent PB MRD monitoring using highly sensitive flow cytometry with a detection limit of one CLL cell in 100,000leukocytes (0·001%).
Results
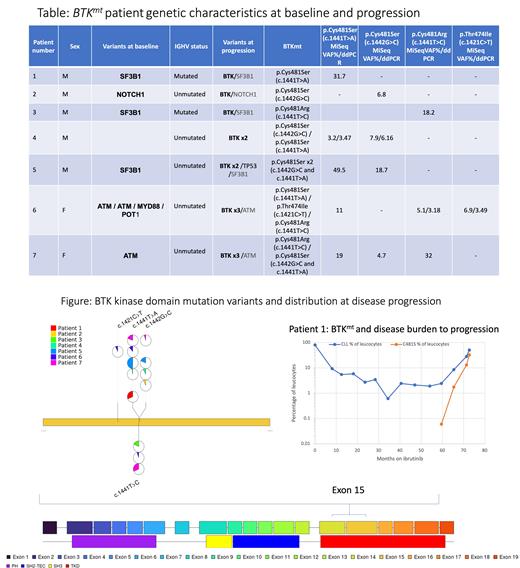
61/386 pts progressed after a median follow up of 44 mo. on I with 50/61 pts progressing after >24 mo. No deaths were recorded before DP. 47/61 IR DP samples were sequenced by MiSeq, 10 further samples with low disease burden were only analysed by ddPCR for hotspot BTKmt. 7/47 sequenced at baseline and DP, developed new isolated mutations in ATM, SF3B1, NOTCH1, BIRC3, BRAF, KLF2, CDKNB1 or TRAF2. The most frequently mutated gene at DP was BTK. From 57/61 sequenced samples at DP, 7 were found to have hotspot BTKmt most with multiple mutations (Fig/Table). No BTKmt detected at DP were detected at baseline by NGS or ddPCR. One pt had a PLCG2 mutation at DP (VAF 33.3%). The 8 BTKmt/ PLCG2 mt IR progressions were mostly in late DP pts (median 60.3 mo.) compared to a median time to DP of 54.3 mo in BTKwt/ PLCG2wt pts. The majority of BTKmt IR DP (5/7) were reported after stopping therapy at 72 mo. 6 monthly samples during I were studied for BTKmt. Evolution of BTKmt in the context of increasing disease burden on I, shows a steady rise in the VAF preceding frank DP (Fig). 148/386 (38.3%) IR pts were 100% homologous to germline IGHV unmutated and 31/148 (20.9%) progressed. 12/15 pts, who acquired additional mutations at DP were IGHV unmutated and 9/12 were 100% homologous to germline including 6/8 with acquired BTK/PLCG2 mutations.
Conclusion
In upfront IR treated standard risk CLL pts 15.8% progressed (61/386), the majority late. Only 13% of the IR DP cohort (8/61) had a BTK/PLCG2 mutation, suggesting other non- BTK/PLCG2 mutations or disease related factors as contributors to DP after initial response in the upfront setting. Interestingly, the background mutation profile did not change from baseline in those who acquired BTK/PLCG2 mutations at DP, but 7/15 acquired non- BTK/ PLCG2mutations at DP. The majority of IR DP with BTK/PLCG2 mutations (6/8), were 100% homologous to germline IGHV unmutated, suggesting an inherent higher risk disease profile as a risk factor for acquiring BTKmt with prolonged BTKi therapy. BTK/PLCG2 mutations were enriched amongst very late progressors with 6/8 pts progressing shortly after stopping therapy at 72 mo., which is later than previously reported for relapsed/refractory CLL pts.
Disclosures
Rawstron:Abbvie: Honoraria, Research Funding; Beigene: Consultancy, Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Pharmacyclics: Consultancy, Research Funding; Gilead: Research Funding. Cairns:Sanofi: Research Funding; Celgene BMS: Honoraria, Research Funding; Janssen: Honoraria; Amgen: Research Funding; Takeda: Research Funding. Hockaday:Abbvie: Speakers Bureau. Bloor:Abbvie: Consultancy, Honoraria, Speakers Bureau; Gilead, Janssen: Honoraria. Munir:Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Hillmen:Apellis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal